Blog
Full spectrum CBD contains natural components of the plant that work together to relieve pain, inflammation, improve sleep, mood and reduce stress and anxiety. MedEcho products support the body's recovery, promote emotional balance and improve quality of life.
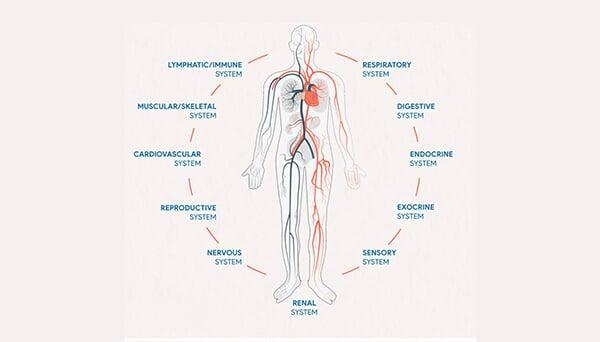
CBD works by interacting with your endocannabinoid system. This system, also known as the ECS is a key homeostatic regulator, which plays a vital role in almost every physiological system in the human body. The endocannabinoid system is best explained as a vast network of receptors that receives che...
CBD stands for Cannabidiol – the main compound found abundantly in hemp plants. Cannabidiol is just one of over 80 natural plant compounds found in this species. Hemp is not only a rich source of Cannabidiol, it also contains other phytocannabinoids, that add to the benefits of using hemp oil produc...
Discover the workings and benefits of CBD edibles at MedEcho CBD. Explore how they work and uncover their potential benefits. Visit us now.
Discover the benefits of water soluble CBD capsules at MedEcho CBD. Enhance absorption and effectiveness for optimal results.
Categories
- About MedEcho
(3)
- CBD for Pain
(1)
- CBD for Sleep
(1)
- CBD for Stress
(1)
- CBD Capsules
(8)
- CBD Chewables
(1)
- Learn
(2)